Welcome to the Gene Expression Laboratory at the Salk Institute
Dr. Geoffrey M. Wahl is a Professor at the Salk Institute, an Adjunct Professor at the University of California, San Diego in the Department of Biology, and the past President of the American Association for Cancer Research (2006-2007). His research focuses on a number of important problems related to cancer biology.
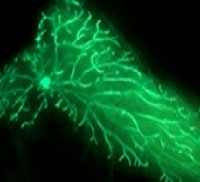 The first project involves identifying, isolating, and characterizing the cells that initiate cancer. His lab’s strategy is to develop molecular-genetic approaches in the mouse to facilitate identification of stem cells in the embryo that are responsible for organ development. Currently, the studies revolve around the mammary gland to determine whether breast cancer arises from mutations within the stem cells that generate this tissue. The long-term goal of these studies is to determine whether such cells exhibit growth requirements and patterns of drug sensitivity that might be targeted for the development of more effective therapeutics. The results from their studies thus far are consistent with the idea that these fetal mammary stem cells share significant similarities with cells found in aggressive breast cancers, and that the molecular signatures of the fetal cells are useful for predicting therapeutic responses.
The first project involves identifying, isolating, and characterizing the cells that initiate cancer. His lab’s strategy is to develop molecular-genetic approaches in the mouse to facilitate identification of stem cells in the embryo that are responsible for organ development. Currently, the studies revolve around the mammary gland to determine whether breast cancer arises from mutations within the stem cells that generate this tissue. The long-term goal of these studies is to determine whether such cells exhibit growth requirements and patterns of drug sensitivity that might be targeted for the development of more effective therapeutics. The results from their studies thus far are consistent with the idea that these fetal mammary stem cells share significant similarities with cells found in aggressive breast cancers, and that the molecular signatures of the fetal cells are useful for predicting therapeutic responses.
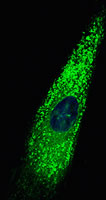 The second project concerns pancreatic cancer, one of the most lethal cancers to afflict humans. Here, the problem is that these cancers are often detected late, and that they develop a very dense and hard to penetrate outer layer of fibrotic tissue. This outer layer creates a type of protective barrier for the cancer cells that reduces the ability of cancer therapies from getting to the cancer cells. The cells in the outer protective layer are called stellate cells, and they normally participate in the response of the pancreas to wounds and inflammation. However, the pancreatic cancer cells produce substances that activate the stellate cells, cause them to divide, and generate fibrosis. Conversely, the activated stellate cells produce substances that promote cancer cell growth and survival. The approach the lab is taking to develop more effective pancreatic cancer therapies is to treat this cancer from the outside in, and from the inside out. To attack the activated stellate cells, they are taking advantage of the expertise they have built in the biology of the p53 tumor suppressor gene over the past twenty years. Their hypothesis is that by activating p53 in the stellate cells, they will be able to stop them from proliferating, and reprogram their biology to be more normal. To attack the cancers from the inside out, they are collaborating with Dr. Andy Lowy and his group at UCSD Moores Cancer Center as they have developed a new strategy to sensitize the cells to chemotherapeutic drugs.
The second project concerns pancreatic cancer, one of the most lethal cancers to afflict humans. Here, the problem is that these cancers are often detected late, and that they develop a very dense and hard to penetrate outer layer of fibrotic tissue. This outer layer creates a type of protective barrier for the cancer cells that reduces the ability of cancer therapies from getting to the cancer cells. The cells in the outer protective layer are called stellate cells, and they normally participate in the response of the pancreas to wounds and inflammation. However, the pancreatic cancer cells produce substances that activate the stellate cells, cause them to divide, and generate fibrosis. Conversely, the activated stellate cells produce substances that promote cancer cell growth and survival. The approach the lab is taking to develop more effective pancreatic cancer therapies is to treat this cancer from the outside in, and from the inside out. To attack the activated stellate cells, they are taking advantage of the expertise they have built in the biology of the p53 tumor suppressor gene over the past twenty years. Their hypothesis is that by activating p53 in the stellate cells, they will be able to stop them from proliferating, and reprogram their biology to be more normal. To attack the cancers from the inside out, they are collaborating with Dr. Andy Lowy and his group at UCSD Moores Cancer Center as they have developed a new strategy to sensitize the cells to chemotherapeutic drugs.
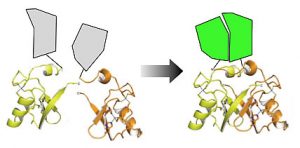 The third project involves the development and use of a new technology to detect and analyze protein interactions and their functional consequences in living cells. This project has enormous potential to understand the pathways that control growth in normal cells, to investigate how cancer mutations perturb growth signaling, and to screen for new drugs that interfere with these pathways by disrupting protein-protein interactions. We have already used this strategy to detect interactions between proteins that have not been amenable to analysis in living cells previously, such as those involving transient and weak interactions. We have also been using this system to study how the Ras protein functions, as this protein is mutated in >90% of human pancreatic cancers.
The third project involves the development and use of a new technology to detect and analyze protein interactions and their functional consequences in living cells. This project has enormous potential to understand the pathways that control growth in normal cells, to investigate how cancer mutations perturb growth signaling, and to screen for new drugs that interfere with these pathways by disrupting protein-protein interactions. We have already used this strategy to detect interactions between proteins that have not been amenable to analysis in living cells previously, such as those involving transient and weak interactions. We have also been using this system to study how the Ras protein functions, as this protein is mutated in >90% of human pancreatic cancers.
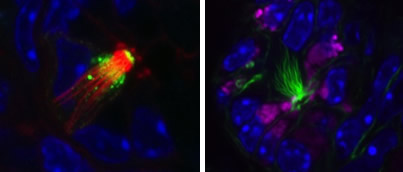 The fourth project concerns pancreatic tumorigenesis and the aberrant pancreatic lesions that precede cancer development. When normal pancreatic tissue undergoes metaplasia there is great heterogeneity in the resulting metaplastic cells, including a significant number of tuft cells, with the potential to direct tissue healing or tumorigenesis. Tuft cells are solitary chemosensory cells found throughout the hollow organs of the digestive tract. They are analogous to taste cells, but do not aggregate in buds. They do, however express proteins consistent with taste, inflammatory, and neuronal cell signaling. Our goal is to determine how tuft cells, and metaplastic heterogeneity, contribute to pancreatic disease and cancer formation. We are currently using genetically engineered mouse models, histology, electron microscopy, RNA sequencing, and primary organoid, fibroblast, and macrophage cultures to answer this question.
The fourth project concerns pancreatic tumorigenesis and the aberrant pancreatic lesions that precede cancer development. When normal pancreatic tissue undergoes metaplasia there is great heterogeneity in the resulting metaplastic cells, including a significant number of tuft cells, with the potential to direct tissue healing or tumorigenesis. Tuft cells are solitary chemosensory cells found throughout the hollow organs of the digestive tract. They are analogous to taste cells, but do not aggregate in buds. They do, however express proteins consistent with taste, inflammatory, and neuronal cell signaling. Our goal is to determine how tuft cells, and metaplastic heterogeneity, contribute to pancreatic disease and cancer formation. We are currently using genetically engineered mouse models, histology, electron microscopy, RNA sequencing, and primary organoid, fibroblast, and macrophage cultures to answer this question.
Together, these projects should shed light on the cells that initiate cancer, how they arise, the conditions that lead to their persistence and perpetuation, and they offer the promise for the development of new therapies to treat diverse malignancies.
 Bianca Lundien Kennedy, the lab’s very special breast cancer advocate who inspires them to work harder to detect breast cancer earlier, and to develop more effective, personalized treatments.
Bianca Lundien Kennedy, the lab’s very special breast cancer advocate who inspires them to work harder to detect breast cancer earlier, and to develop more effective, personalized treatments.